Sign up to our free Living Well email for advice on living a happier, healthier and longer life Live your life healthier and happier with our free weekly Living Well newsletter
Hospitals in the UK are trialling a new vaccine which prevents children from developing chickenpox.
Vaccines for the illness are currently available in Germany and the US, but is not included in the UK’s routine childhood vaccination programme, and only offered to vulnerable groups.
The NHS website explains that it is not routine in the UK due to fears it could cause chickenpox and shingles in older people, which can lead to more serious complications.
The study will test how safe and effective a new version of a vaccine called Varilrix is, and will involve children aged between 12 and 23 months.
Scientists estimate that the vaccine, which was licensed in the UK in 2013, gives 98 per cent protection against chicken pox in children and 75 per cent in adolescents and adults.
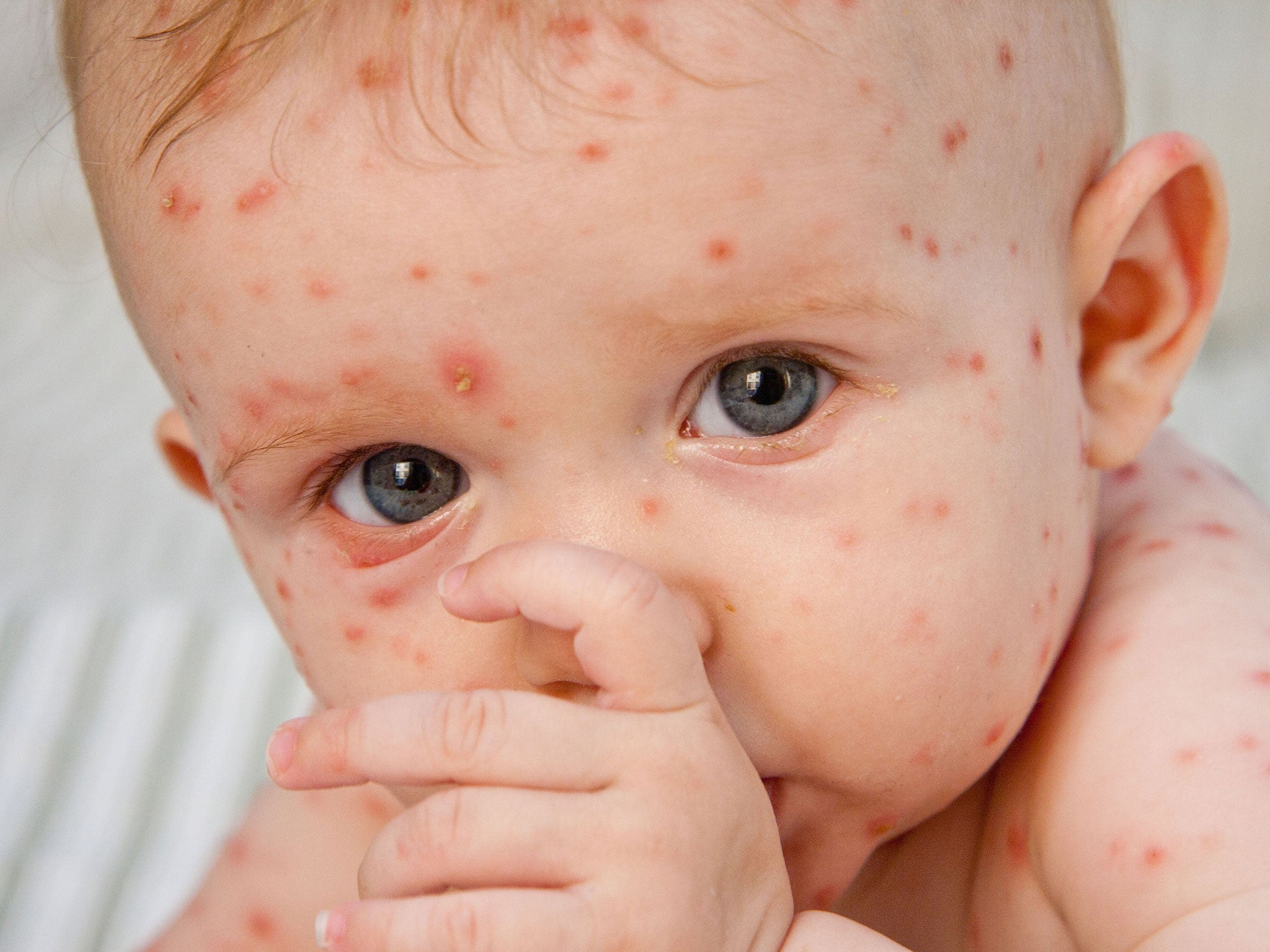
Chickenpox, caused by the varicella-zoster virus, is a common and relatively mild condition which lasts between five to 10 days.
Symptoms include a red rash, and itchy spots that turn into blisters.
However, in vulnerable children and adults, it can cause serious conditions such as pneumonia, skin infections, and brain swelling, known as encephalitis.
The study is being conducted at the NIHR Wellcome Trust Southampton Clinical Research Facility and St George's Hospital in south London, as well as sites in Bristol and Oxford.
Show all 40 1 /40Health news in pictures Health news in pictures Coronavirus outbreak The coronavirus Covid-19 has hit the UK leading to the deaths of two people so far and prompting warnings from the Department of Health
AFP via Getty
Health news in pictures Thousands of emergency patients told to take taxi to hospital Thousands of 999 patients in England are being told to get a taxi to hospital, figures have showed. The number of patients outside London who were refused an ambulance rose by 83 per cent in the past year as demand for services grows
Getty
Health news in pictures Vape related deaths spike A vaping-related lung disease has claimed the lives of 11 people in the US in recent weeks. The US Centre for Disease Control and Prevention has more than 100 officials investigating the cause of the mystery illness, and has warned citizens against smoking e-cigarette products until more is known, particularly if modified or bought “off the street”
Getty
Health news in pictures Baldness cure looks to be a step closer Researchers in the US claim to have overcome one of the major hurdles to cultivating human follicles from stem cells. The new system allows cells to grow in a structured tuft and emerge from the skin
Sanford Burnham Preybs
Health news in pictures Two hours a week spent in nature can improve health A study in the journal Scientific Reports suggests that a dose of nature of just two hours a week is associated with better health and psychological wellbeing
Shutterstock
Health news in pictures Air pollution linked to fertility issues in women Exposure to air from traffic-clogged streets could leave women with fewer years to have children, a study has found. Italian researchers found women living in the most polluted areas were three times more likely to show signs they were running low on eggs than those who lived in cleaner surroundings, potentially triggering an earlier menopause
Getty/iStock
Health news in pictures Junk food ads could be banned before watershed Junk food adverts on TV and online could be banned before 9pm as part of Government plans to fight the "epidemic" of childhood obesity. Plans for the new watershed have been put out for public consultation in a bid to combat the growing crisis, the Department of Health and Social Care (DHSC) said
PA
Health news in pictures Breeding with neanderthals helped humans fight diseases On migrating from Africa around 70,000 years ago, humans bumped into the neanderthals of Eurasia. While humans were weak to the diseases of the new lands, breeding with the resident neanderthals made for a better equipped immune system
PA
Health news in pictures Cancer breath test to be trialled in Britain The breath biopsy device is designed to detect cancer hallmarks in molecules exhaled by patients
Getty
Health news in pictures Average 10 year old has consumed the recommended amount of sugar for an adult By their 10th birthdy, children have on average already eaten more sugar than the recommended amount for an 18 year old. The average 10 year old consumes the equivalent to 13 sugar cubes a day, 8 more than is recommended
PA
Health news in pictures Child health experts advise switching off screens an hour before bed While there is not enough evidence of harm to recommend UK-wide limits on screen use, the Royal College of Paediatrics and Child Health have advised that children should avoid screens for an hour before bed time to avoid disrupting their sleep
Getty
Health news in pictures Daily aspirin is unnecessary for older people in good health, study finds A study published in the New England Journal of Medicine has found that many elderly people are taking daily aspirin to little or no avail
Getty
Health news in pictures Vaping could lead to cancer, US study finds A study by the University of Minnesota's Masonic Cancer Centre has found that the carcinogenic chemicals formaldehyde, acrolein, and methylglyoxal are present in the saliva of E-cigarette users
Reuters
Health news in pictures More children are obese and diabetic There has been a 41% increase in children with type 2 diabetes since 2014, the National Paediatric Diabetes Audit has found. Obesity is a leading cause
Reuters
Health news in pictures Most child antidepressants are ineffective and can lead to suicidal thoughts The majority of antidepressants are ineffective and may be unsafe, for children and teenager with major depression, experts have warned. In what is the most comprehensive comparison of 14 commonly prescribed antidepressant drugs to date, researchers found that only one brand was more effective at relieving symptoms of depression than a placebo. Another popular drug, venlafaxine, was shown increase the risk users engaging in suicidal thoughts and attempts at suicide
Getty
Health news in pictures Gay, lesbian and bisexual adults at higher risk of heart disease, study claims Researchers at the Baptist Health South Florida Clinic in Miami focused on seven areas of controllable heart health and found these minority groups were particularly likely to be smokers and to have poorly controlled blood sugar
iStock
Health news in pictures Breakfast cereals targeted at children contain 'steadily high' sugar levels since 1992 despite producer claims A major pressure group has issued a fresh warning about perilously high amounts of sugar in breakfast cereals, specifically those designed for children, and has said that levels have barely been cut at all in the last two and a half decades
Getty
Health news in pictures Potholes are making us fat, NHS watchdog warns New guidance by the National Institute for Health and Care Excellence (NICE), the body which determines what treatment the NHS should fund, said lax road repairs and car-dominated streets were contributing to the obesity epidemic by preventing members of the public from keeping active
PA
Health news in pictures New menopause drugs offer women relief from 'debilitating' hot flushes A new class of treatments for women going through the menopause is able to reduce numbers of debilitating hot flushes by as much as three quarters in a matter of days, a trial has found. The drug used in the trial belongs to a group known as NKB antagonists (blockers), which were developed as a treatment for schizophrenia but have been “sitting on a shelf unused”, according to Professor Waljit Dhillo, a professor of endocrinology and metabolism
REX
Health news in pictures Doctors should prescribe more antidepressants for people with mental health problems, study finds Research from Oxford University found that more than one million extra people suffering from mental health problems would benefit from being prescribed drugs and criticised “ideological” reasons doctors use to avoid doing so.
Getty
Health news in pictures Student dies of flu after NHS advice to stay at home and avoid A&E The family of a teenager who died from flu has urged people not to delay going to A&E if they are worried about their symptoms. Melissa Whiteley, an 18-year-old engineering student from Hanford in Stoke-on-Trent, fell ill at Christmas and died in hospital a month later.
Just Giving
Health news in pictures Government to review thousands of harmful vaginal mesh implants The Government has pledged to review tens of thousands of cases where women have been given harmful vaginal mesh implants.
Getty
Health news in pictures Jeremy Hunt announces 'zero suicides ambition' for the NHS The NHS will be asked to go further to prevent the deaths of patients in its care as part of a “zero suicide ambition” being launched today
Getty
Health news in pictures Human trials start with cancer treatment that primes immune system to kill off tumours Human trials have begun with a new cancer therapy that can prime the immune system to eradicate tumours. The treatment, that works similarly to a vaccine, is a combination of two existing drugs, of which tiny amounts are injected into the solid bulk of a tumour.
Nephron
Health news in pictures Babies' health suffers from being born near fracking sites, finds major study Mothers living within a kilometre of a fracking site were 25 per cent more likely to have a child born at low birth weight, which increase their chances of asthma, ADHD and other issues
Getty
Health news in pictures NHS reviewing thousands of cervical cancer smear tests after women wrongly given all-clear Thousands of cervical cancer screening results are under review after failings at a laboratory meant some women were incorrectly given the all-clear. A number of women have already been told to contact their doctors following the identification of “procedural issues” in the service provided by Pathology First Laboratory.
Rex
Health news in pictures Potential key to halting breast cancer's spread discovered by scientists Most breast cancer patients do not die from their initial tumour, but from secondary malignant growths (metastases), where cancer cells are able to enter the blood and survive to invade new sites. Asparagine, a molecule named after asparagus where it was first identified in high quantities, has now been shown to be an essential ingredient for tumour cells to gain these migratory properties.
Getty
Health news in pictures NHS nursing vacancies at record high with more than 34,000 roles advertised A record number of nursing and midwifery positions are currently being advertised by the NHS, with more than 34,000 positions currently vacant, according to the latest data. Demand for nurses was 19 per cent higher between July and September 2017 than the same period two years ago.
REX
Health news in pictures Cannabis extract could provide ‘new class of treatment’ for psychosis CBD has a broadly opposite effect to delta-9-tetrahydrocannabinol (THC), the main active component in cannabis and the substance that causes paranoia and anxiety.
Getty
Health news in pictures Over 75,000 sign petition calling for Richard Branson's Virgin Care to hand settlement money back to NHS Mr Branson’s company sued the NHS last year after it lost out on an £82m contract to provide children’s health services across Surrey, citing concerns over “serious flaws” in the way the contract was awarded
PA
Health news in pictures More than 700 fewer nurses training in England in first year after NHS bursary scrapped The numbers of people accepted to study nursing in England fell 3 per cent in 2017, while the numbers accepted in Wales and Scotland, where the bursaries were kept, increased 8.4 per cent and 8 per cent respectively
Getty
Health news in pictures Landmark study links Tory austerity to 120,000 deaths The paper found that there were 45,000 more deaths in the first four years of Tory-led efficiencies than would have been expected if funding had stayed at pre-election levels. On this trajectory that could rise to nearly 200,000 excess deaths by the end of 2020, even with the extra funding that has been earmarked for public sector services this year.
Reuters
Health news in pictures Long commutes carry health risks Hours of commuting may be mind-numbingly dull, but new research shows that it might also be having an adverse effect on both your health and performance at work. Longer commutes also appear to have a significant impact on mental wellbeing, with those commuting longer 33 per cent more likely to suffer from depression
Shutterstock
Health news in pictures You cannot be fit and fat It is not possible to be overweight and healthy, a major new study has concluded. The study of 3.5 million Britons found that even “metabolically healthy” obese people are still at a higher risk of heart disease or a stroke than those with a normal weight range
Getty
Health news in pictures Sleep deprivation When you feel particularly exhausted, it can definitely feel like you are also lacking in brain capacity. Now, a new study has suggested this could be because chronic sleep deprivation can actually cause the brain to eat itself
Shutterstock
Health news in pictures Exercise classes offering 45 minute naps launch David Lloyd Gyms have launched a new health and fitness class which is essentially a bunch of people taking a nap for 45 minutes. The fitness group was spurred to launch the ‘napercise’ class after research revealed 86 per cent of parents said they were fatigued. The class is therefore predominantly aimed at parents but you actually do not have to have children to take part
Getty
Health news in pictures 'Fundamental right to health' to be axed after Brexit, lawyers warn Tobacco and alcohol companies could win more easily in court cases such as the recent battle over plain cigarette packaging if the EU Charter of Fundamental Rights is abandoned, a barrister and public health professor have said
Getty
Health news in pictures 'Thousands dying' due to fear over non-existent statin side-effects A major new study into the side effects of the cholesterol-lowering medicine suggests common symptoms such as muscle pain and weakness are not caused by the drugs themselves
Getty
Health news in pictures Babies born to fathers aged under 25 have higher risk of autism New research has found that babies born to fathers under the age of 25 or over 51 are at higher risk of developing autism and other social disorders. The study, conducted by the Seaver Autism Center for Research and Treatment at Mount Sinai, found that these children are actually more advanced than their peers as infants, but then fall behind by the time they hit their teenage years
Getty
Health news in pictures Cycling to work ‘could halve risk of cancer and heart disease’ Commuters who swap their car or bus pass for a bike could cut their risk of developing heart disease and cancer by almost half, new research suggests – but campaigners have warned there is still an “urgent need” to improve road conditions for cyclists. Cycling to work is linked to a lower risk of developing cancer by 45 per cent and cardiovascular disease by 46 per cent, according to a study of a quarter of a million people. Walking to work also brought health benefits, the University of Glasgow researchers found, but not to the same degree as cycling.
Getty
Katrina Cathie, a consultant paediatrician and principal investigator for the study at Southampton Children's Hospital, said her team is “extremely pleased” to be part of the “exciting trial.”
“While chickenpox is often a mild illness which lasts for one or two weeks, it can still be very uncomfortable and unpleasant for children while, in the worst cases - particularly among those with underlying health conditions - it can lead on to respiratory infection, skin infection and brain inflammation.”
She explained that the study will investigate whether a new version of the vaccine should be developed, by monitoring how the temperature of participants change, as well as signs of fever.
"Through this study, we will find out if a new version of the vaccine is better than the current version," she said.
Additional reporting by PA

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies