Mole tester app 'deceptively' claiming to analyse cancer risk without evidence, says watchdog
The Mole Detective app allowed smartphone users to photograph suspicious moles over a period of weeks or months and calculate a 'risk factor'
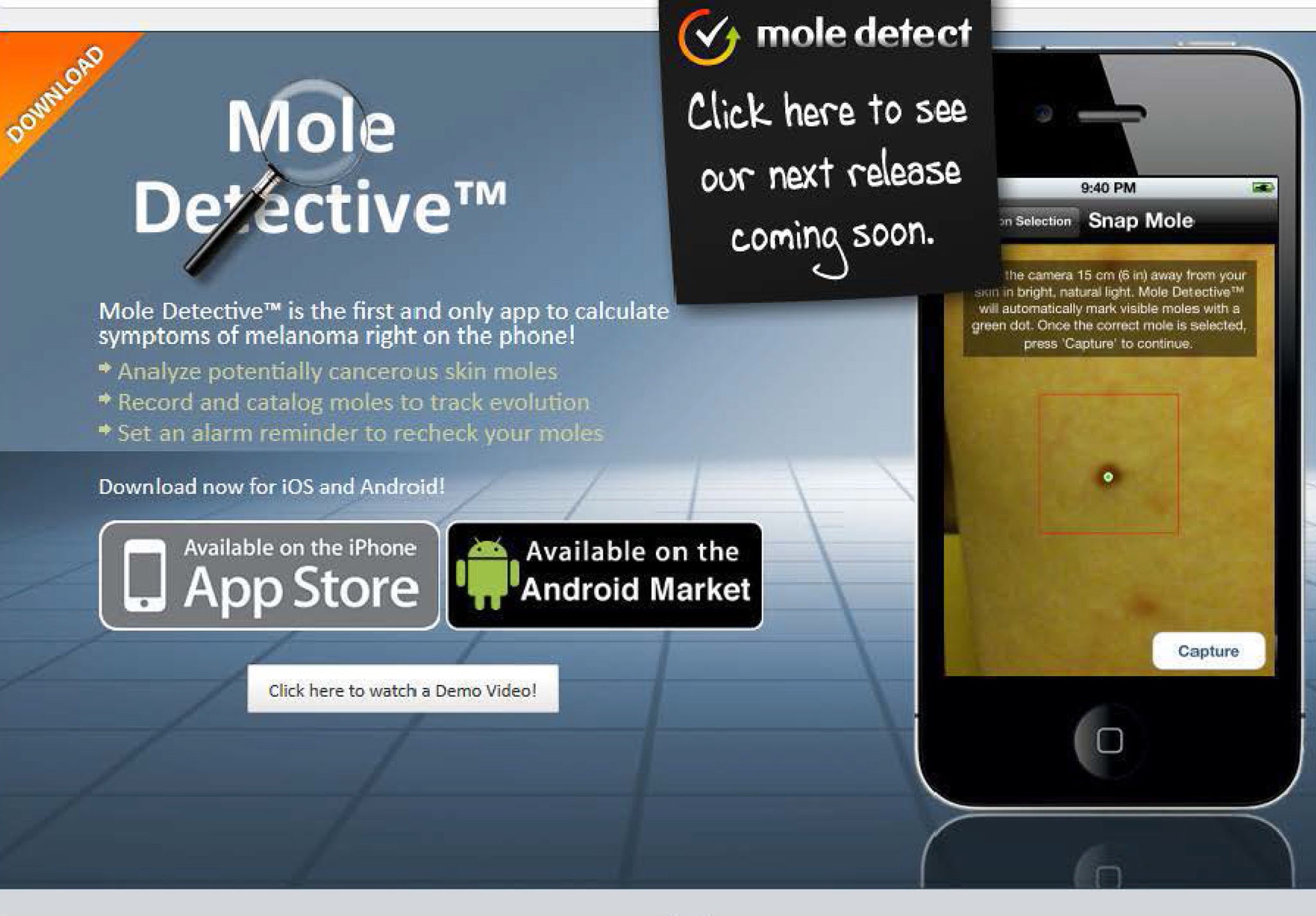
For those worried about skin cancer, the Mole Detective app must have seemed a very modern way of checking whether a worrisome freckle was a cause for concern.
Marketed and owned by a British health company, the app allowed smartphone users to photograph suspicious moles over a period of weeks or months and, using its software, calculate a “risk factor” on whether a lesion merited professional investigation.
The programme, available via Apple and Google for $4.99 (£3.40), was just one of some 97,000 health apps now in circulation. Some 20 per cent of Britons are already using a health or fitness-related app, with the NHS launching its own library of approved apps.
The Federal Trade Commission (FTC) in Washington, however, saw things differently. Now the British company behind the app, L Health Ltd, and its entrepreneur owner, Avi Lasarow, are being pursued in the American courts for alleged breach of consumer protection rules through its advertising.
The watchdog claims the app was “deceptively” marketed by claiming accurately to analyse a cancer risk without the necessary supporting evidence and is seeking potentially hefty fines against its UK backers. In the meantime, Mole Detective has been taken offline.
The case, which is being fought by Mr Lasarow, throws up serious questions about the rapidly escalating use of health apps to help treat conditions from depression to diabetes .
The Independent can reveal that the prosecution against Mole Detective, first marketed by Mr Lasarow’s company in August 2012, has split the FTC in America with one of the four experts required to authorise the proceedings saying it should not go ahead and it risks “chilling” the sector’s development.
Maureen Ohlhausen, an FTC commissioner, accused the organisation of making an “unduly expansive interpretation” of advertisers’ claims, and of failing to credit consumers with the ability to differentiate between an app and medical expertise.
In a written statement, she said: “I fear this course of action will inhibit the development of beneficial products and chill the dissemination of useful health information.”
The FTC insisted that Mr Lasarow and his company had nonetheless infringed the rules by claiming in marketing material that the app could “increase the chance of detecting skin cancer in early stages”. The watchdog said it had seen no scientific evidence to prove the effectiveness of Mole Detective, which claimed to offer an electronic version of the same assessment techniques used by professional dermatologists.
Mr Lasarow said he had bought the app from its American developer in good faith 2012 and questioned whether regulators were up to speed with the changing demands of consumers.
He said: “The app stated that it should be used for educational purposes only and not replace a doctor visit. It is difficult for the regulators to keep up. But it is certainly necessary that they devise an approach that is helpful to developers and investors alike, while assuring adequate protection of consumers.”
In Britain, the Medicines and Healthcare Products Regulatory Agency warned app developers this week it was looking for apps which cause harm and asked patients to report all adverse effects. The agency requires all medical devices, including apps, to carry a “CE” certification meeting European standards.
Neil McGuire, the watchdog’s clinical director of devices, said: “If you have a medical device and it’s software or an app and patients come to grief, we’re coming looking.”
An app a day... how the NHS is embracing smartphone technology
From portable blood-pressure monitors which link with an app to send readings to clinicians to a miniaturised blood testing device which uses another app to detect exposure to diseases including HIV, health professionals agree that smartphone technology has the potential transform detection of health problems.
The ability to use an app to constantly monitor data for sufferers of conditions from asthma to heart disease could also have a transformative effect on the management of chronic conditions.
The NHS has drawn up a list of 268 apps for a growing online “library” which has been assessed by experts to be helpful in areas from insomnia to quitting smoking.
There is early evidence that the accessibility of an app on a phone or tablet, especially when it provides a direct link to carers or fellow sufferers, could be particularly effective when dealing with mental illness.
A library of mental illness apps was unveiled by the NHS last week in the belief that it will help those who are reluctant to access help via more traditional means such as their GP. One service, Big White Wall, which offers anonymised access to professional support, said some 80 per cent of respondents felt able to self-manage their condition after using it.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies