Wrinkle treatments should be regulated, says British Association of Aesthetic Plastic Surgeons
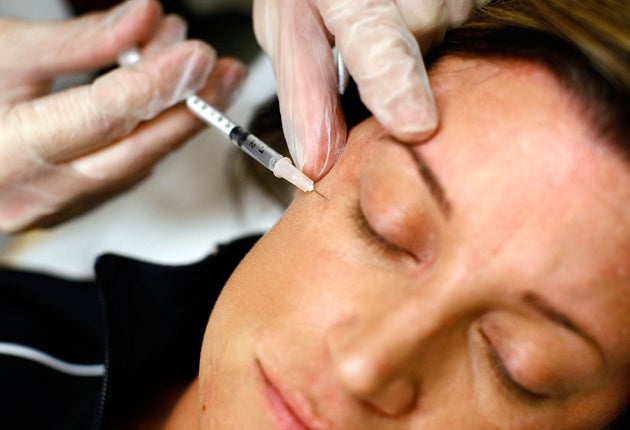
Injectable anti-wrinkle treatments should be classed as medicines so they can be properly regulated, the British Association of Aesthetic Plastic Surgeons (Baaps) said.
Baaps has recently raised concerns that people can buy dermal filler treatments online without knowing exactly what chemicals are in them.
The organisation suggests that the treatments, which involve injecting a gel-like substance into wrinkle sites, should be classed as medicines so that they are properly tested before they can be sold.
The move would also regulate who can give the treatments and would ban advertising for the products.
Baaps president Rajiv Grover said: "It is of paramount importance that the growing area of largely unregulated dermal fillers be controlled and we have put forward for consideration the simple measure of reclassifying these injectables as medicines.
"This will kill three birds with one stone by regulating which ones come on the market, automatically banning their advertising and defining who is qualified to dispense them."
The body made the recommendation to the Keogh review of cosmetic surgery and procedures.
Baaps also suggested that the term 'surgeon' is legally protected and that there should be a compulsory register for practitioners.
Surgeons from abroad should have UK-based indemnity insurance and advertising for cosmetic procedures should be "severely restricted", a Baaps spokeswoman added.
Baaps said there should be a financial protection scheme for people who choose to have cosmetic surgery to prevent another scenario akin to the PIP implant scandal.
Around 47,000 British women are believed to have been given the implants manufactured by French company Poly Implant Prothese (PIP). They were filled with non-medical grade silicone intended for use in mattresses and have been linked to rupture and swelling in the body.
Mr Grover continued: "The call for evidence by the Keogh review has given our sector an ideal platform to air issues that have long been concerning many of us.
"The PIP implants crisis demonstrated beyond a shadow of a doubt that there are clear failures in the system, as well as vulnerable people being taken advantage of. It may not be possible to prevent criminal activity in the future, but certainly safeguards can be put in place to protect the public as much as possible."
PA
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies