Ebola virus outbreak: First person to be given possible vaccine in Africa following British trials
The NIAID/GSK vaccine was tested at the University of Oxford
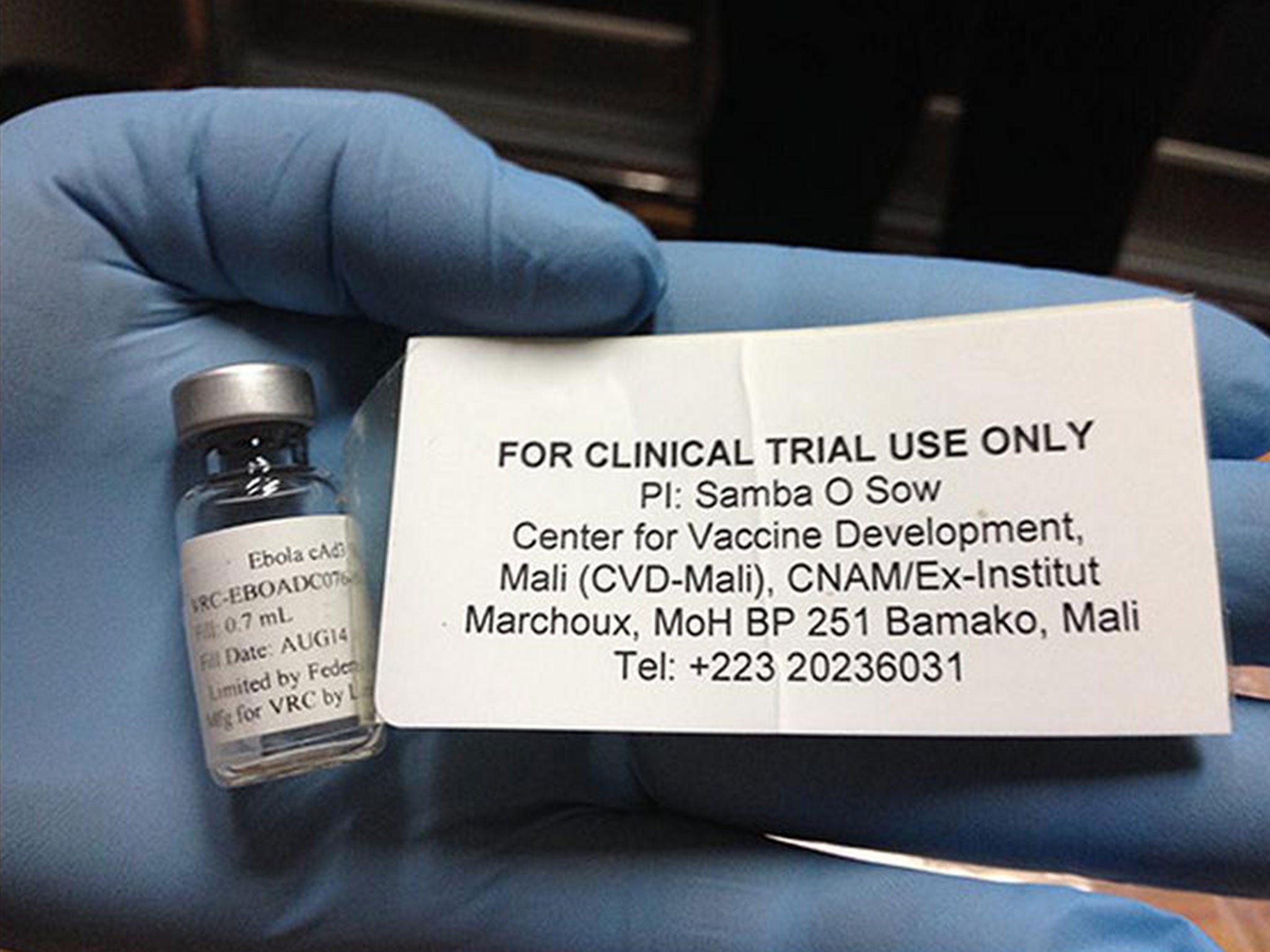
The first trials of a British-tested Ebola vaccine in Africa are due to start on health workers in Mali.
Known as the NIAID/GSK vaccine, it uses a usually harmless cold virus called adenovirus as a carrier for a single Ebola protein.
Developers believe it stimulates the body into building immune defences against the disease but cannot transmit Ebola because it does not contain infectious material.
Trials at the University of Oxford, funded by the Wellcome Trust and the Government, have been promising and British firm GlaxoSmithKline (GSK) is manufacturing more than 10,000 doses of the vaccine.
Professor Myron Levine, director of the Centre for Vaccine Development at the University of Maryland, said the trials in Mali could pave the way for wider use of the vaccine.
“This research will give us crucial information about whether the vaccine is safe, well tolerated and capable of stimulating adequate immune responses in the highest priority target population, health care workers in West Africa,” he said.
“If it works, in the foreseeable future it could help alter the dynamic of this epidemic by interrupting transmission to health care and other exposed front-line workers.”
He is leading the current trials, along with Professor Samba Sow of the Centre for Vaccine Development in Mali.
A total of 40 people are due to be vaccinated in Bamako in the coming weeks once permission has been granted by the relevant authorities.
“The very people who take care of sick patients are now afraid to come to work, afraid of catching the disease themselves and passing it to their families.”
A parallel clinical trial will follow in Gambia, also with 40 volunteers, when permission has been granted from the relevant authorities.
Each set of volunteers will be split into groups of 20, receiving different doses of the vaccine so researchers can evaluate which is best for safety and effectiveness.
Professor Sow said the “critical first step” had been taken but a series of tests still have to be carried out to fully access the vaccine.
“If it is eventually shown to work and if this information can be generated fast enough, it could become a public health tool to bring the current, and future, Ebola virus disease epidemics under control,” he added.
The candidate Ebola vaccine was co-developed by the US National Institutes of Health and GSK to protect against the Zaire strain of Ebola, which is the one circulating in West Africa.
The UK trial, at the Jenner Institute at the University of Oxford, ran alongside similar trials in the USA carried out by the National Institute of Allergy and Infectious Diseases.
Professor Levine said the speed of the project was “a testament to everyone’s commitment to fighting Ebola as aggressively as possible”.
In September, a 48-year-old NHS employee was the first person to be injected with the NIAID/GSK vaccine during the Oxford trials in September.
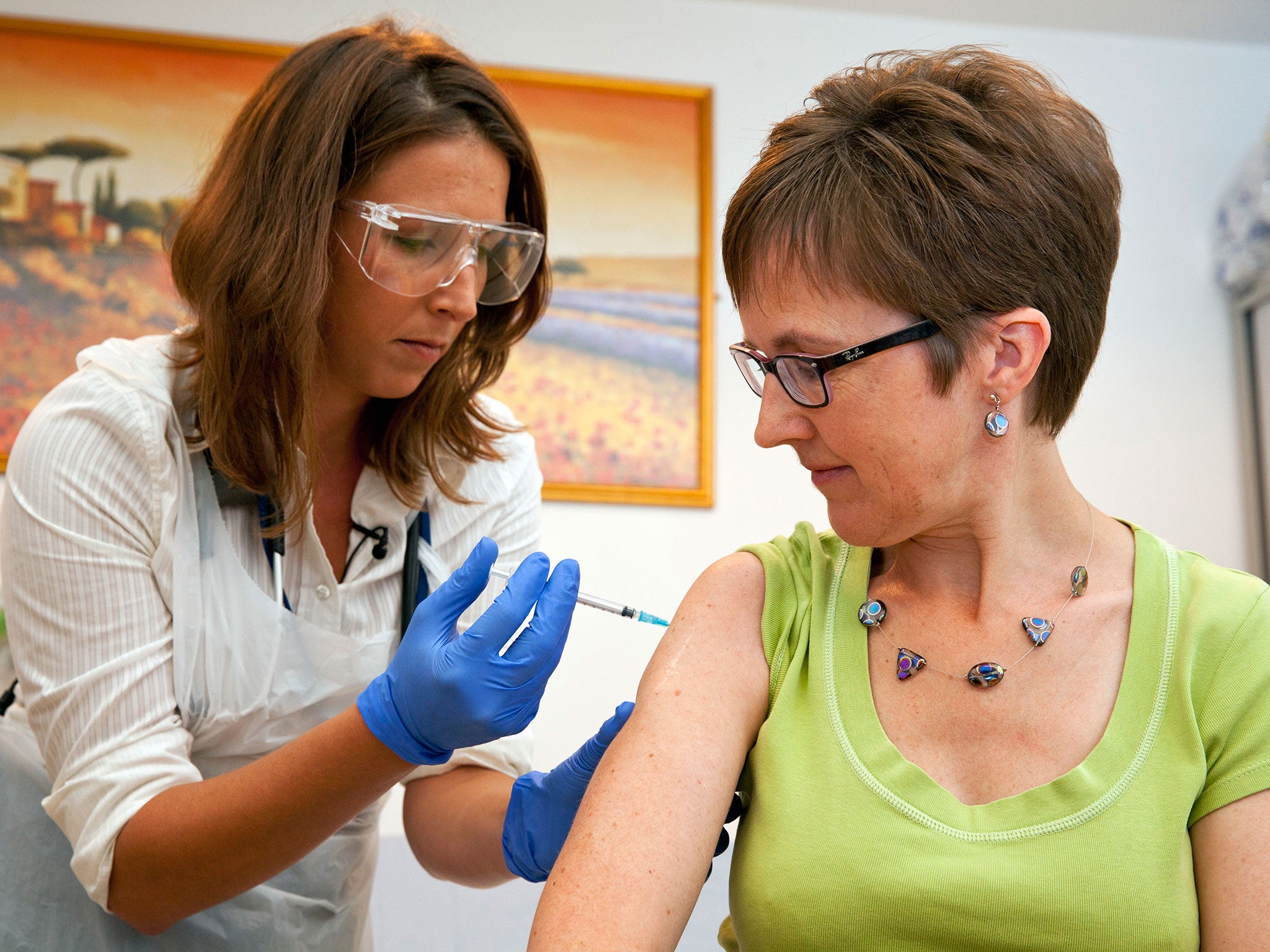
Ruth Atkins, a communications and engagement manager from Oxfordshire, was the first of 60 volunteers to receive it, saying afterwards she felt “absolutely fine”.
She said: “I volunteered because the situation in West Africa is so tragic and I thought being part of this vaccination process was something small I could do to hopefully make a huge impact.”
More than 3,800 people have so far been killed in the Ebola virus outbreak, mainly in Sierra Leone, Liberia and Guinea.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments