Cystic fibrosis: Gene therapy offers hope to patients after successful trials
Scientists said it was the first time in more than 20 years of research on the cystic fibrosis gene that anyone has shown definite advantages of gene therapy
A glimmer of hope for the thousands of families affected by cystic fibrosis has emerged from a clinical trial showing that gene therapy produced a modest but significant improvement among children suffering from the most common inherited disease of white Europeans.
Scientists in Britain said it was the first time in more than 20 years of research on the cystic fibrosis gene that anyone has shown definite advantages of gene therapy - the deliberate attempt to correct the defective gene behind cystic fibrosis, which primarily affects the lungs.
A clinical trial involving 136 patients in the UK has demonstrated a “modest and variable” improvement in lung function without any harmful side-effects as a result of a monthly dose of gene therapy over the course of a year, doctors said.
“For the first time in the world we have seen a significant benefit in giving gene therapy to cystic fibrosis patients,” said Professor Eric Alton, the co-ordinator of the UK consortium of universities and hospitals that carried out the trial.
“Patients who received the gene therapy showed a significant, if modest, benefit in tests of lung function compared with the placebo group; there were no safety concerns,” Professor Alton said.
“Whilst the effect was inconsistent, with some patients responding better than others, the results are encouraging, laying the groundwork for further trials which we hope could improve the effect,” he said.
Doctors are wary of raising expectations among families affected by cystic fibrosis - which affects 10,000 patients in Britain - after repeated claims since the gene was discovered in 1989 that a gene-therapy cure was just around the corner.
However, the members of the UK Cystic Fibrosis Gene Therapy Consortium believe they have now made the key initial breakthrough that allows them to proceed with further clinical trials involving more active forms of the gene-therapy treatment.
“We are looking to undertake follow-up studies assessing higher, more frequent doses as well as combinations with other treatments,” Professor Alton said.
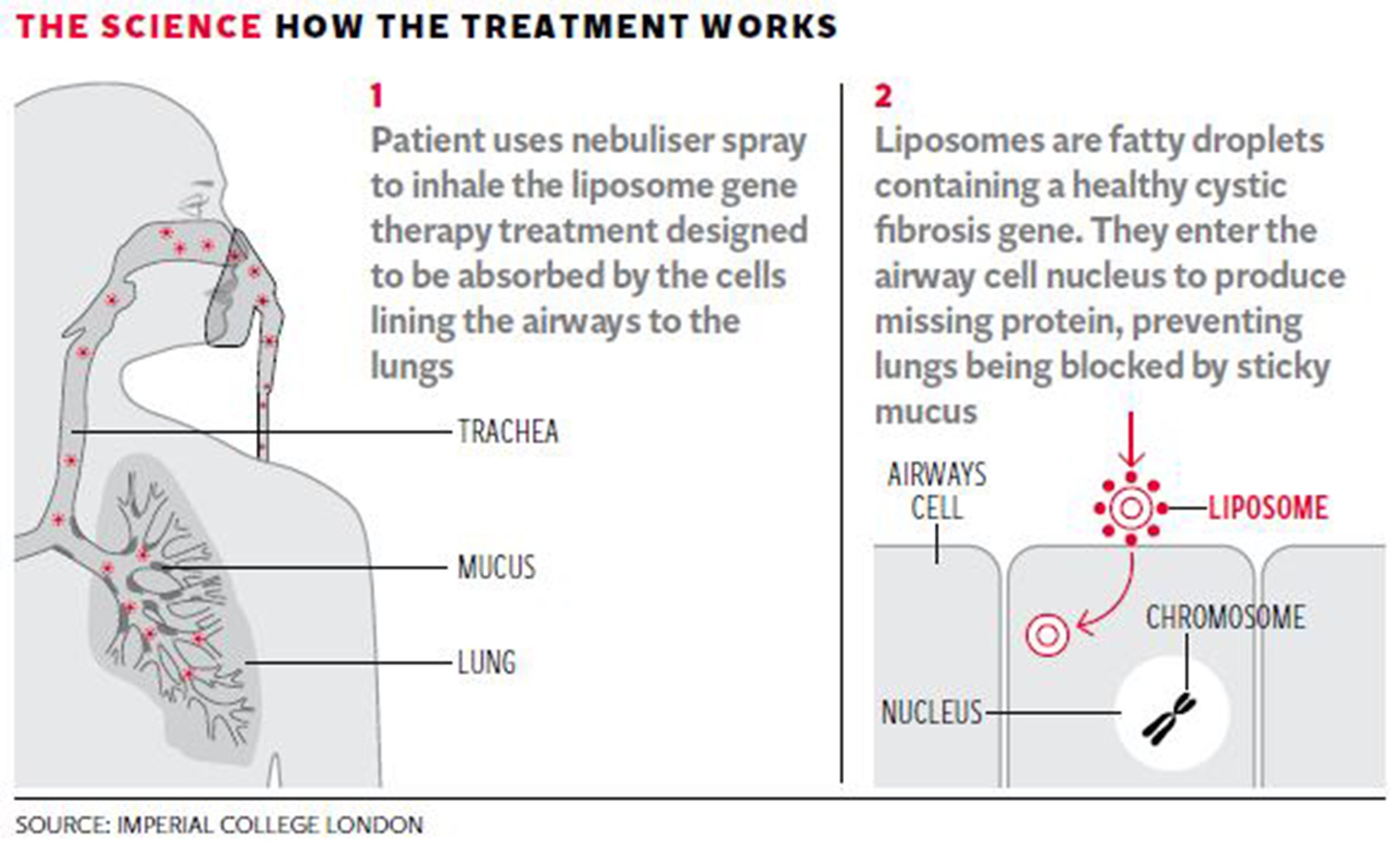
The latest trial involved 40-minute inhalations once a month for year with a nebulising spray containing fatty droplets or “liposomes” wrapped around a healthy, synthetic copy of the cystic fibrosis gene.
The liposomes are designed to be absorbed by the cells lining the airways of cystic fibrosis patients, where the gene stimulates the production of the healthy protein in the cell membranes that prevents the the lungs from clogging with sticky mucus.
Results of the trial published in the journal The Lancet Respiratory Medicine revealed that there was about a 5-per-cent improvement in lung function among the treated patients, which rose to about 6 per cent in the worst-affected sub-group.
The patients were over the age of 12 and had a lung function between 50 per cent and 90 per cent of healthy individuals. Some said they felt better and were able to clear the mucus from their lungs, while others reported no improvement, said Professor Jane Davies of Imperial College, a member of the consortium.
The next phase of the research, which has yet to receive funding, is to increase the liposome dose and to develop a parallel treatment involving a hybrid virus that will be able to insert the healthy copy of the gene directly into the chromosomes of the affected lung cells, Professor Alton said.
“Our aim is to achieve a step change in the treatment of CF that focuses on the basic defect rather than just addressing the symptoms. It has taken more than 20 years to get where we are now, and there is still some way to go,” he said.
The £3m phase-2 trial took place at the Royal Brompton Hospital in London and the Western General Hospital in Edinburgh, involving scientists from the universities of Oxford, Edinburgh and Imperial College London funded by the Medical Research Council, the National Institute for Health Research and the Cystic Fibrosis Trust.
Ed Owen of the trust said: “This is an extraordinary time for therapeutic development in cystic fibrosis and the need is urgent to stop so many young lives being cut short because of this cruel condition.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments