Rise of acid ocean eats away base of food chain
Shells of tiny sea snails are being eroded as more carbon dioxide is dissolved into seawater
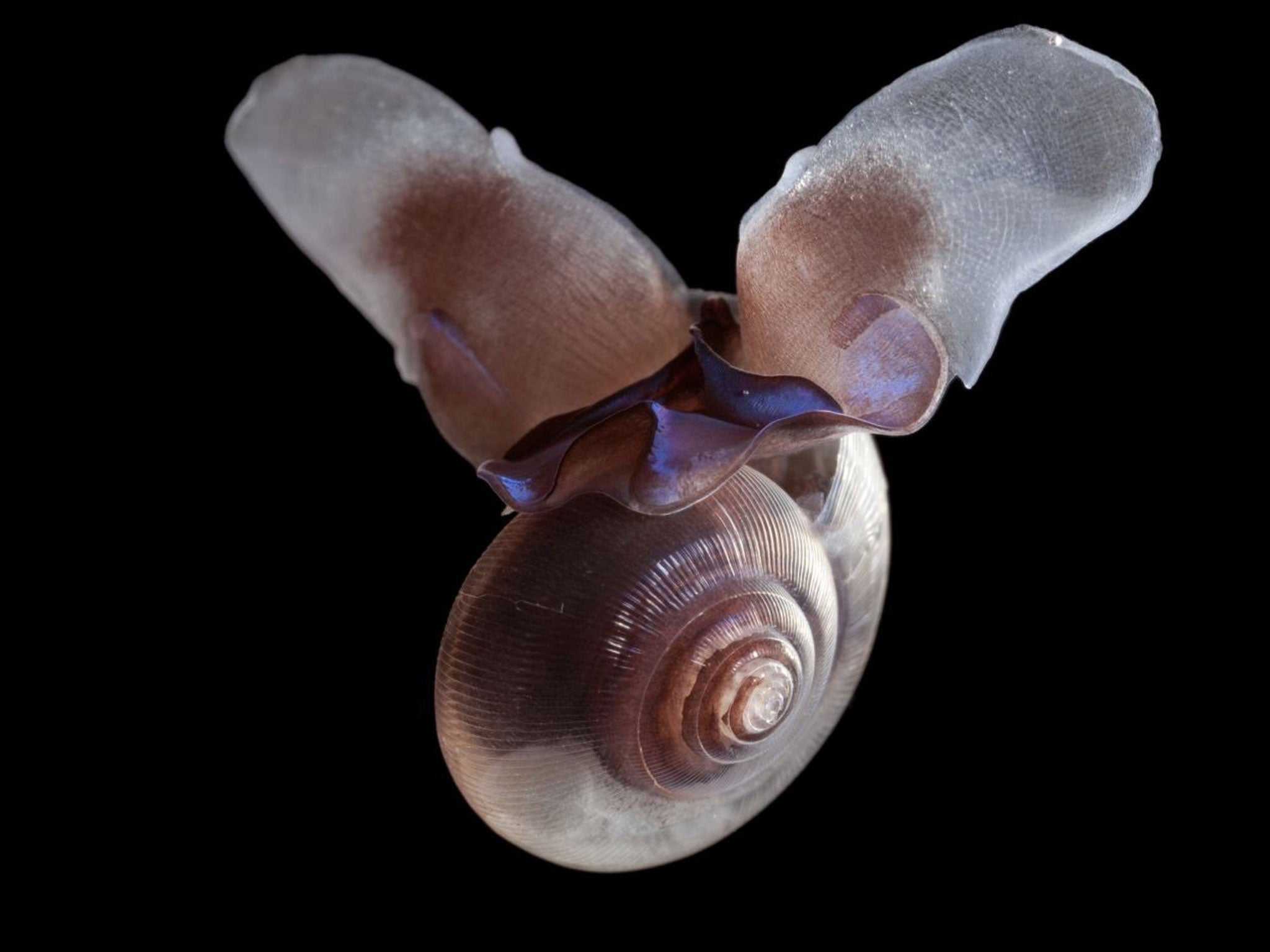
Rising amounts of carbon dioxide dissolving in the ocean is causing the acid corrosion of tiny sea creatures that form the base of the marine food chain, scientists have discovered.
Ocean acidification caused by increased levels of carbon dioxide in the atmosphere is eating away at the shells of marine snails known as “sea butterflies”, the researchers said.
It is the first time that scientists have discovered the visibly acid-damaged shells of critically-important organisms living in the Southern Ocean off Antarctica. The researchers believe it could be a harbinger of worse things to come.
The sea butterflies, also known as pteropod snails, live in the surface layers of the open ocean, grow no bigger than a centimetre across and are part of the floating plankton on which all other fish and marine animals ultimately depend for their survival.
“Pteropods are an important food source for fish and birds as well as a good indicator of ecosystem health,” said Geraint Tarling of the British Antarctic Survey in Cambridge.
“The tiny snails do not necessarily die as a result of their shells dissolving, however it may increase their vulnerability to predation and infection, consequently having an impact on other parts of the food web,” Dr Tarling said.
In 2008, scientists on board a British scientific research vessel collected samples of pteropod snails from the Scotia Sea in the Atlantic sector of the Southern Ocean.
A microscopic analysis of a random sample of live pteropods revealed extensive acid erosion of their shells. It is the first documented case of acid damage to the shells of living wild pteropods, Dr Tarling said.
The scientists also found that the surrounding seawater had relatively low concentrations of a critically important calcium mineral called aragonite which the pteropods need for shell making. Aragonite concentrations fall when the seawater becomes less alkaline – and more acidic.
“The corrosive properties of the water caused shells of live animals to be severely dissolved and this demonstrates how vulnerable pteropods are,” said Nina Bednarsek, formerly of the British Antarctic Survey and now at the US National Oceanic and Atmospheric Administration.
“Ocean acidification resulting from the addition of human-induced carbon dioxide contributed to this dissolution,” said Dr Bednarsek, the lead author of the study, published in the journal Nature Geoscience.
The researchers found the damaged snails over areas of ocean “upwelling”, where cold, nutrient-rich deep water rises to the surface. Pteropods and other plankton congregate over upwelling spots because they are good sources of nutrients and food.
Upwelling is known to have a corrosive effect on marine shells because deep seawater is naturally rich in dissolved carbon dioxide and so, when it rises to the surface, it lowers the concentration of aragonite in the shallower layers of the ocean where pteropods live.
However, the researchers found that the increased concentration of carbon dioxide in the atmosphere, caused by the burning of fossil fuels, has tipped the aragonite balance in favour of the acidic corrosion of the pteropod shells in these upwelling areas.
“We know that the seawater becomes more corrosive to aragonite shells below a certain depth…which occurs around 1,000 metres depth,” Dr Bednarsek said.
“However, at one of our sampling sites, we discovered that this point was reached at 200 metres depth, through a combination of natural upwelling and ocean acidification. Marine snails – pteropods – live in this top layer of the ocean,” she said.
Dr Tarling said that computer modelling identified the role played by man-made carbon dioxide in the atmosphere which is causing the acidification of the oceans.
“If we had the carbon dioxide concentrations we had a century or more ago, the conditions wouldn’t have got to the corrosive state that we have observed,” said Dr Tarling.
If carbon dioxide concentrations continue to rise as expected in the coming decades, the areas of the ocean that will become corrosive to shelled creatures will spread over much wider areas, he added.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments