Exclusive: Silicone implant risk 'eight times greater'
Fresh evidence suggests thousands more women face problems as Health Secretary orders review after cosmetic surgery firm says 1 in 12 reports faults
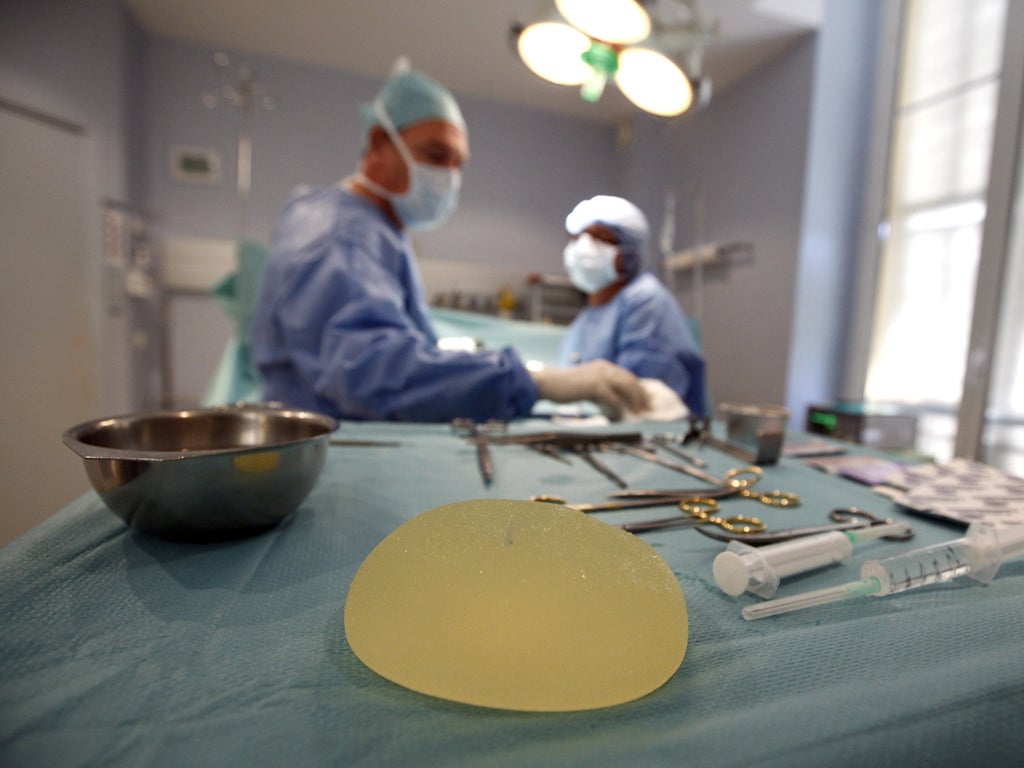
One in 12 of the 40,000 British women who have received faulty breast implants containing industrial silicone is at risk of developing serious medical complications, The Independent on Sunday has learned.
Andrew Lansley, the Health Secretary, ordered an urgent review yesterday into the quality of data about the proportion of women who have had problems with the French-made Poly Implants Prothèses (PIP) implants at the centre of the scandal.
The IoS can exclusively reveal the investigation was triggered by a leading cosmetic surgery firm, which privately warned ministers that the proportion of women at risk is as high as 8 per cent, rather than the 1 per cent previously claimed in the UK.
The company is responsible for about 4,000 of the UK's PIP implants, but if an 8 per cent rupture rate proves accurate and is widely replicated, it could mean a total of 3,200 women are affected. In France, where authorities shut down PIP last year, the risk of rupture is said to be about 5 per cent, and women have been advised to have the implants removed – a move backed by British surgeons but not, so far, by ministers.
But if the risk rate is confirmed as being substantially higher in the UK than in France, pressure will grow for the Department of Health to follow the French Health Minister, Xavier Bertrand, in urging women to have them removed. Abnormal rupture rates of the implants, which contain industrial-strength silicone commonly used in mattresses, emerged in 2009. Symptoms can include lumps around the implant or in the under-arm, inflammation in the breast tissue and a hardening of the breast.
"Our priority is making sure that women get the correct advice so that they are kept safe," Mr Lansley said, as he announced that the NHS medical director, Professor Sir Bruce Keogh, will review the data given to the UK's Medicines and Healthcare products Regulatory Agency (MHRA).
An emergency report is expected on Mr Lansley's desk early this week. The MHRA will audit all data held on the quality of the implants, including information from private clinics, which are responsible for about 95 per cent of implant procedures.
More than 250 British women are said to be suing the clinics that treated them. Mr Lansley said: "I want to reassure women that if any new data calls into question the safety of these implants, we will act swiftly to help them." He highlighted concerns about the "content and quality of the data that cosmetic surgery providers are sharing with the regulator".
Last night, surgeons accused the MHRA of downplaying the true extent of the problem and called for an implant register to track when things go wrong. The MHRA is now considering such a move. If British women are advised to have the PIP implants removed, the NHS could be left to pick up the bill as many of the clinics responsible are no longer in business.
Fazel Fatah, president of the British Association of Aesthetic Plastic Surgeons, said: "[It could be] better to take them out now rather than wait for them to rupture, which makes the procedure more complicated. We think the French decision is not unreasonable at all."
Andy Burnham, the shadow Health Secretary, said: "The Government should provide more advice and support to women who may be affected by this announcement. I have heard reports that some women are experiencing delays or difficulty in accessing records from private cosmetic surgery companies, or in some cases large fees. That is unacceptable."
Dr Susanne Ludgate, clinical director of the MHRA, said concerns had been raised by "conflicting data" coming from the cosmetic surgery industry. "It raises doubts about the surveillance and reporting of incidents by these companies. We will urgently work to identify where problems may be," she added.
Paul Balen, of Freeth Cartwright solicitors, has spent years representing British women who have been fitted with PIP implants. The implant company came to the attention of the MHRA more than a decade ago, when concerns over its hydrogel implants led to them being recalled in 2000.
Peter Walsh, from the patient safety charity Action against Medical Accidents (AvMA), said there can be "no doubt that the systems of regulation which are supposed to protect patients have already been shown to be wanting". The AvMA is now calling for the Commons Health Select Committee to investigate.
Additional reporting by Melanie Newman
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies