The Big Question: Has a key breakthrough been made in the search for a cure for cancer?
Why are we asking this now?
British scientists announced yesterday that they have sequenced a "cancer genome" for the first time. It means they have identified all of the many thousands of genetic mistakes that make a tumour cell different from a healthy cell taken from the same cancer patient.
Not all of these mistakes, or DNA mutations, were involved in triggering the cancer, but some of them – the "drivers" – clearly were. Scientists believe it will be possible eventually to identify these driver mutations and find the genetic faults that led to the changes in a healthy human cell that caused it to divide uncontrollably to form a cancerous tumour.
How could this lead to a possible cure for cancer?
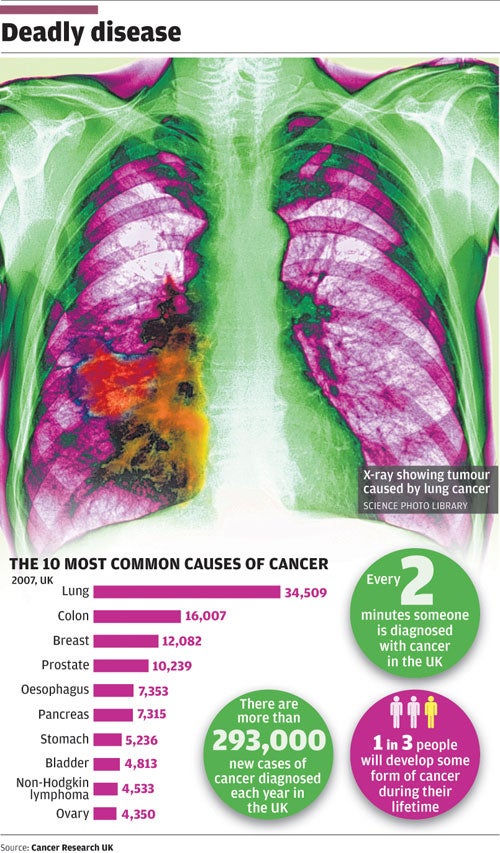
There is unlikely ever to be a single "cure" for cancer, which after all affects so many different tissues and organs of the body. In fact, there may be as many as 200 different types of cancer, and many more subtypes. But each and every cancer involves damage to the DNA template that rules the cell and governs the way it divides. In this respect, cancer is a genetic disease, indeed it is said to be the most common genetic disease since, in the developed world, it strikes one in three people over a lifetime, killing as many as one in five.
By understanding the nature of these genetic mutations in a cancer cell, it should be possible to design tailor-made drugs that specifically target the faults, or the outcome of the faults. It could also lead to new methods of diagnosing cancer in the earliest stages of the disease before it becomes apparent to the patient or doctor, or new ways of finding secondary cancers lurking in the body that have evaded earlier anti-cancer treatment.
Who carried out this work?
It was a team of human genome scientists led by Peter Campbell and Professor Mike Stratton at the Sanger Institute near Cambridge, which is funded by the Wellcome Trust, the world's biggest medical research charity. They are part of the International Cancer Genome Consortium, a collaboration of research institutes from countries such as Britain and France in Europe, the United States, Canada, Japan and Australia. They will be taking samples from about 500 patients around the world in the hope of analysing the genomes of the 50 most common cancers.
What was actually done in the latest study?
The Sanger Institute scientists analysed cells stored from two patients who had died of cancer. One was a 55-year-old man with small-cell lung cancer and the other was a 45-year-old man with malignant melanoma, the most lethal form of skin cancer. The researchers took a cancerous cell and a healthy cell from each patient and sequenced the full genetic code, or genome, of all four cells. They did this dozens of times over to make sure they had a correct final sequence, consisting of some 3 billion letters of the full human genome.
And what was the result?
The scientists found that the lung cancer cell had 22,910 DNA mutations that the healthy cell from the same patient did not possess. These mutations in the lung must have accumulated during the lifetime of the patient, many as a result of exposure to cigarette smoke. The same goes for the 33,345 mutations identified in the cancerous skin cells of 45-year-old man with malignant melanoma, although most of the mutations here are presumed to have been caused by exposure to sunlight.
Both of these "cancer genomes" show where the mutations occurred and in which of the chromosomes of the cell. They were published in the journal Nature. It was the scientific first step towards the "personalised medicine" of sequencing the DNA of cancer patients on a routine basis.
What do these mutations look like?
Some of them involve quite big changes to the DNA molecule, such as rearrangements of hundreds of thousands of letters in the four-letter code of DNA. But some of them are the smallest change possible, a shift for instance in one letter (known as a base) to another, such as C to T and vice versa, or an A to G and vice versa. These "base pairs" are at the heart of the DNA sequencing exercise.
Some of these mutations are already known from previous studies to be linked with certain environmental mutagens, the mutation-causing agents. Tobacco smoke, for instance, often results in the mutation of G to T, whereas ultraviolet light tends to mutate C to T. By looking at the mutations in the lung-cancer cell and the skin-cancer cell, scientists were able to see the influence that smoking and exposure to the sun had had on the DNA of these two patients. "In the melanoma sample, we can see sunlight's signature writ large in the genome," said Andy Futreal at the Sanger Institute.
But not all the mutations would have been involved in triggering the cancer. Most of them would have been harmless "passenger" mutations, but some of them would have been "drivers" within the genes that are in some way involved in cancer development.
How can this be used to identify the 'driver' mutations that cause cancer?
For this, it would be necessary to extend the sequencing effort into other patients suffering from the same cancer, perhaps as many as 500 people to achieve statistical significance. By comparing all mutations in all patients with the same cancer, scientists will be able to identify those that appear to be common to them all, and hence likely to be involved in triggering that particular disease.
Scientists have already identified more than 30 genes that play some kind of role in cancer development. This gives them a lead in terms of knowing where to search for the likely driver mutations that are probably involved in causing the cancer.
How might this lead to the development of new anti-cancer drugs?
In the past, cancer drugs were discovered largely by trial and error. Now it is possible to find the precise genetic fault that causes a cell to divide uncontrollably and so hopefully be able to design a drug that can fix that specific fault.
For instance, scientists found that faults in a gene called BRAF were involved in triggering a high proportion of skin cancers. The mutations meant that the BRAF gene was permanently switched in the "on" position, causing the cells to divide continually in malignant melanoma. Scientists are now developing drugs that turn this gene "off", and some of these substances are near to clinical trials.
What do the experts say about this work?
They are very excited by it – they have branded it "remarkable", "groundbreaking" and "fascinating". But it will still be many years before we can expect full genome sequencing of a patient's cells to be used routinely in hospitals and clinics – that is if the NHS can ever afford it given the parlous state of public finances.
Should we be optimistic about future cancer treatments?
Yes...
* This represents a transformation in our understanding of the genetic faults at the heart of cancer
* DNA sequencing technology is getting faster and cheaper and will one day be routine
* Finding the driver mutations of cancer will open the door to new drugs and diagnostic tools
No...
* It is still going to be many years before these developments will be used routinely on cancer patients
* The NHS can barely afford existing anti-cancer treatments and 'personalised medicine' will cost even more
* History tells us that the 'war on cancer' is never-ending, and there are many more battles to be fought
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks