One death and dozens of infections linked to contaminated eye gel, health chiefs say
52 patients have developed infections due to an outbreak of antibiotic resistant bacteria
One person has died and dozens of others have been infected after using a contaminated gel used to treat dry eyes, health chiefs have suggested.
The UK Health Security Agency said there had been 52 confirmed and six probable cases linked to an outbreak of a bacteria called Burkholderia cepacia complex, with 38 people needing critical care.
Experts also believe the bacteria “contributed to the death of one case” during the outbreak, which took place between January 2023 and February this year. The youngest person affected was a baby while the oldest was aged 91, the report said.
Investigators looked at any links between the bacteria outbreak and the use of specific brands of eye gel, which are available over the counter or online.
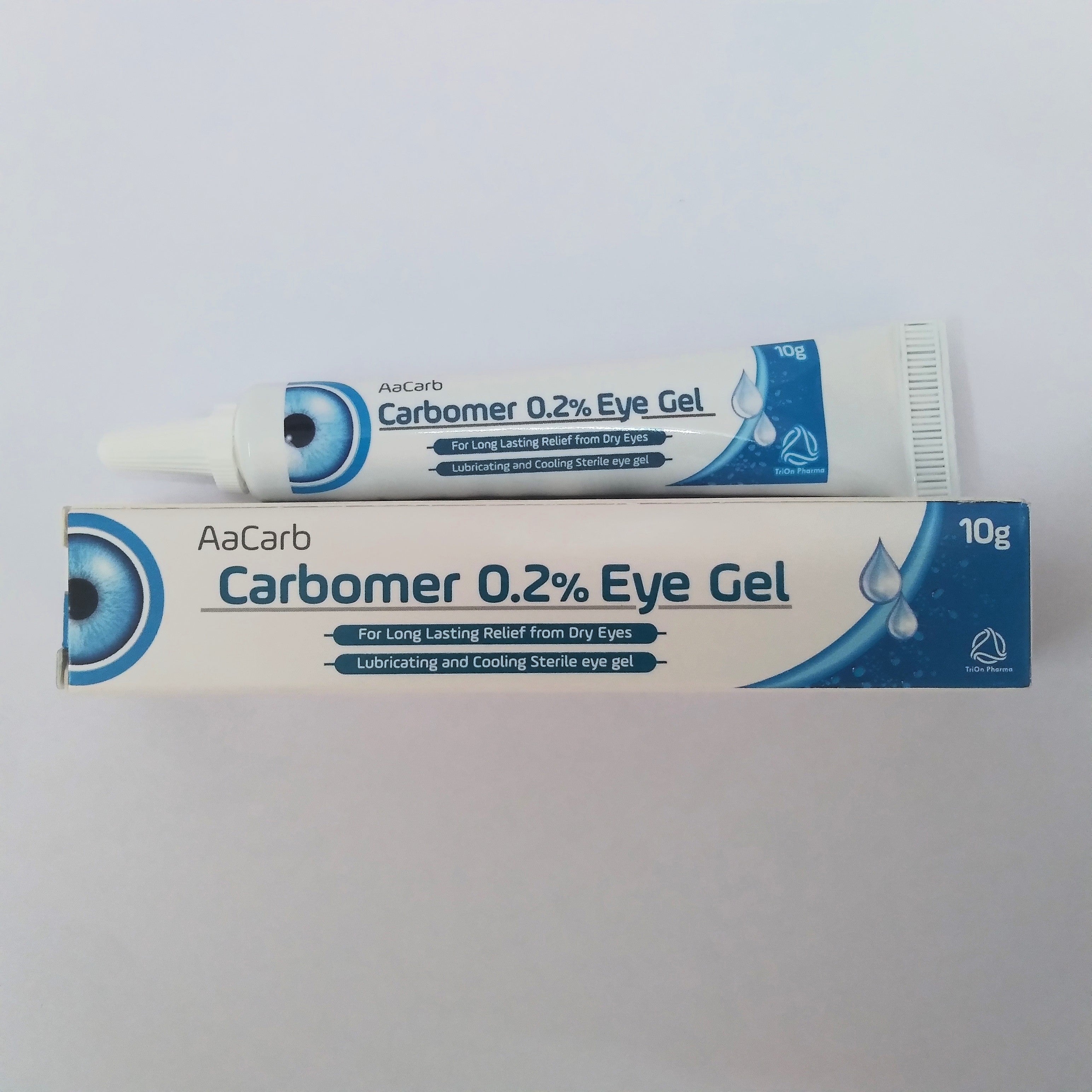
The Medicines and Healthcare products Regulatory Agency (MHRA), the medicines watchdog, also issued a safety notice on certain batches of carbomer-containing gel in November “due to possible microbiological contamination”. At the same time, certain batches of three eye gels were recalled.
According to the notice, a manufacturer called Indiana Ophthalmics LLP issued an alert to withdraw and recall 19 batches of its eye gel products, called AaCarb, Aacomer and Puroptics.
The UKHSA report highlights how the bacteria widely found within the environment, such as soil and water, but is naturally resistant to many antibiotics.
It “very rarely causes infection” among healthy people but can cause severe infection among people with weakened immune systems and people with cystic fibrosis, the report stated.
Around seven out of 10 cases, 71 per cent, were among patients in hospital.
The MHRA said it has now received “sufficient assurance from manufacturers and suppliers to conclude that products available on the UK market are safe to use and free of contamination”.
“UKHSA will continue to follow up new cases and keep vigilance for emergent clusters of Burkholderia cepacia complex,” the report said.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks