Orkambi: Family of girl with cystic fibrosis launch legal challenge over lack of NHS funding for breakthrough drug
Treatment costs more than £100,000 a year but can slow the rate of lung damage by half potentially giving many more years of active life
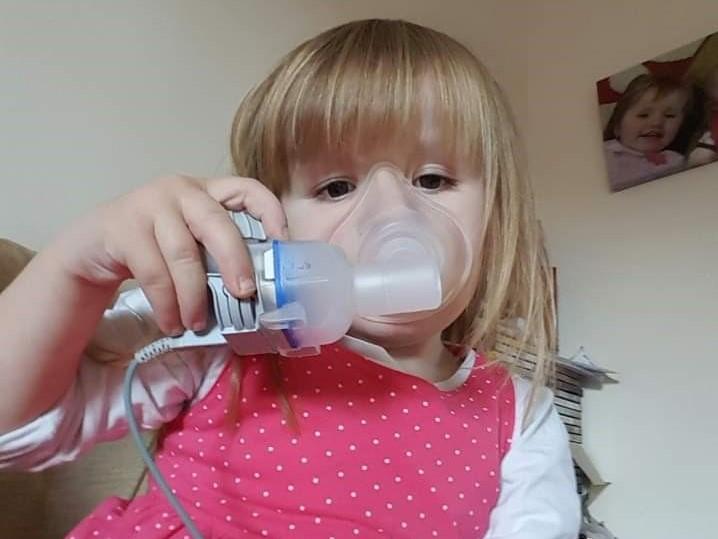
The family of a six-year-old girl with cystic fibrosis who was denied funding for a breakthrough drug which could extend her life has launched a judicial review against the NHS.
Despite her consultant recommending the precision drug Orkambi, Katie Stafford was told by the NHS that it was only available in “exceptional circumstances” because of its high price tag. The drug costs £104,000 per patient for a year of treatment.
Katie’s mother Sarah Burgwin said it was “shameful” that it was being withheld when it could prevent her lungs and quality of life deteriorating.
“It is heart-breaking to see Katie suffer when I know there is a drug out there that could help prevent her torment,” she said. “They are putting a price on the life of my daughter. It’s shameful, what gives them the right to play God with my child’s life?”
Katie, who lives with her family in Totnes, Devon, also has learning and behavioural disabilities that make managing the genetic condition.
Cystic fibrosis (CF) is a genetic condition which causes stick mucus to build up in the lungs of sufferers, increasing their chances of serious lung infections and over time causing scarring that makes it harder to get oxygen.
The average life expectancy for someone born with the condition in the UK is around 47 and many patients may require a lung transplant in their lifetime.

There are over 10,000 CF sufferers in the UK.
Orkambi has been hailed as a breakthrough drug which could benefit around half of British patients with the condition – according to the Cystic Fibrosis Trust.
Clinical trials have shown it can slow the decline of lung function by as much as 46 per cent and it is available in several European countries.
However, NHS bosses have been engaged in years of negotiations with Orkambi’s manufacturer Vertex Pharmaceuticals to secure an affordable deal.
This is despite the government’s claims that the company was offered the “largest ever” financial commitment in the history of the NHS with a five year £500m deal to make the drug available.
At present the National Institute for Health and Care Excellence (Nice), which decides which treatments should be available on the NHS in England and Wales, says it is too expensive for the health service to provide routinely.
Ms Burgwin and her solicitors believe that Katie does meet the exceptional circumstances requirements of the NHS as she has a learning disability which means other treatments are not available.

She said: “I hope that we can win this legal battle, not just for Katie, but also so this can be a gateway for other children also getting this drug to make their quality of life so much better.”
Peter Todd, a partner at Hodge Jones and Allen, the firm launching the judicial review against the NHS decision, said: “Thousands of parents have been left in a desperate position of watching their children deteriorate with this life-shortening condition, while knowing that there is a drug out there that can help improve their health and extend their lives.”
Nick Medhurst, head of public affairs at the Cystic Fibrosis Trust said that orkambi should be available "to all who can benefit". In the instance of people with another condition, such as autism, he said: "The Trust believes that Orkambi should be used routinely and not exceptionally as it is proved to offer significant improvement above standard care."
A NHS England spokesperson said: “Orkambi for the treatment of cystic fibrosis is not recommended by NICE and therefore is not routinely commissioned. Individual funding requests for treatments that are not routinely commissioned are difficult decisions, which is why they are taken by experienced teams on the basis of clinical evidence.”
Earlier this year, health ministers wrote to the company urging it to drop Orkambi’s price, saying “time was of the essence” for cystic fibrosis patients.
A petition signed by more than 100,000 people also triggered a debate on the issue in Parliament this year.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments