Biggest ovarian cancer breakthrough in a decade hailed by researchers
About a fifth of women with ovarian cancer are diagnosed at a stage when their disease has already spread around the body and is incurable
Researchers have hailed the biggest breakthrough in advanced ovarian cancer for a decade after a new treatment was found to dramatically shrink tumours.
Experts called results of the early-phase study “exciting” and “promising” after patients who had exhausted all other treatment options showed an impressive response.
The drug, known as ONX-0801, was tested in 15 women with advanced ovarian cancer as part of a wider trial run by the Institute of Cancer Research (ICR) and the Royal Marsden NHS Foundation Trust in London.
The aim was to test its safety, but the results were so good that researchers are keen to move the drug to the next stage of research as soon as possible.
In the trial, which is still ongoing, ONX-0801 significantly shrank tumours in seven of the 15 ovarian cancer patients.
In those patients whose tumours had the particular molecular target for the drug, the results were even more impressive, with seven out of 10 women responding.
ONX-0801 is the first in a new class of drugs discovered at the ICR. It attacks ovarian cancer by mimicking folic acid to enter the cancer cells.
The drug then kills these cells by blocking a molecule called thymidylate synthase, thereby causing irreparable DNA damage.
Ovarian cancer cells have an abnormally large number of receptors for folic acid, called alpha folate receptors. This means these cancer cells respond particularly well to the treatment.

Dr Udai Banerji, deputy director of the drug development unit at the ICR and the Royal Marsden, who led the study, said much more research was needed but the results were exciting.
He said: “As this is a completely new mechanism of action it should add upward of six months to patients’ lives with minimal side-effects in extremely late phase ovarian cancer.
“This is much more than anything that has been achieved in the last 10 years.”
He said if clinical trials proved the drug’s effectiveness, it could potentially be used in early-stage disease where “the impact on survival may be better”.
Because the new therapy is so specifically targeted at cancer cells, it leaves healthy cells alone. This means it does not have the side-effects, such as infections, diarrhoea, nerve damage and hair loss, often seen with chemotherapy.
Experts have also created tests to detect the cells that will respond particularly well to the treatment, meaning doctors can identify those women who will benefit the most.
Dr Banerji said: “The results we have seen in this trial are very promising. It is rare to see such clear evidence of reproducible responses in these early stages of drug development.
“The beauty of this particular drug is that it is targeted to the cancer cell. This means there are fewer side-effects, making it a kinder treatment for ovarian cancer patients.
“It’s early days of course, but I’m keen to see this treatment assessed in later-stage clinical trials as soon as possible.”
Professor Paul Workman, chief executive of the ICR, said: “It’s really exciting to see such positive results in an early-stage trial.
“It looks a highly promising treatment with the potential to have huge benefits for women with ovarian cancer, and I’m very keen to see it progress to later-stage trials.”
The symptoms of ovarian cancer can be vague, and about six out of 10 women are diagnosed at a later stage of the disease.
Around a fifth of women with the cancer are diagnosed at a stage when their disease has already spread around the body and is incurable.
There were 7,378 new cases of ovarian cancer in the UK in 2014 and more than 4,000 women died from the disease.
Symptoms can include pain in the abdomen or side, a bloated or full feeling and sometimes back pain, constipation or irregular bleeding.
Overall, only about half of all women diagnosed with ovarian cancer live for five years or more.
The results of the new trial were presented at the American Society of Clinical Oncology (Asco) meeting in Chicago.
Marianne Heath, 68, received the ONX-0801 drug as part of a phase one clinical trial at the Royal Marsden cancer hospital in London.
The Swedish-born mother of three was diagnosed with ovarian cancer in 2011 at an advanced stage three after her GP initially thought she was suffering from irritable bowel syndrome.
Ms Heath has tried several types of chemotherapy but has stopped responding to each one. She started on ONX-0801 last September and had an infusion every two weeks for a total of 12 weeks.
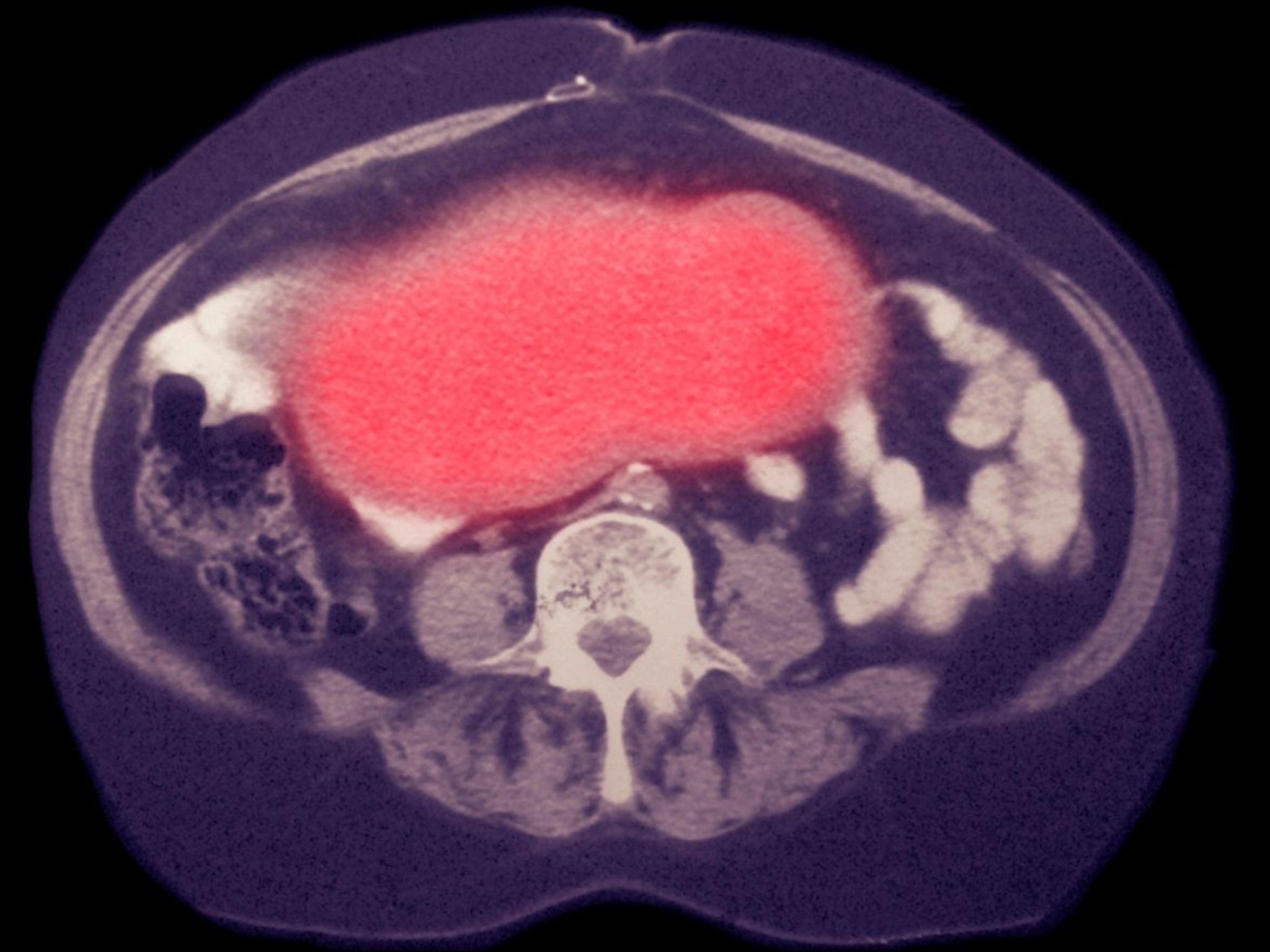
During the trial, all her tumours shrank and she is now considered to have stable disease. However, she has started suffering back pain and plans to have radiotherapy on a tumour in her back.
She said: “It was not a big decision for me to go on a phase one trial as I had no other treatment choices, so I felt this was my only option.
“I just want to keep going so I can keep the tumours at a level where I can enjoy my life. I’m obviously very delighted – I had next to no side-effects. Possibly, if I had to look for a side-effect, I felt tired. But it was very insignificant.
“This has been the best [treatment] so far. It was a very good end result.”
Annwen Jones, chief executive of the charity Target Ovarian Cancer, said: “This study shows promising results, although it is a small sample and at a very early stage of research.
“With very few effective treatment options for ovarian cancer, an approach that develops new ways of targeting ovarian cancer cells more effectively, and with fewer side effects, is to be welcomed.”
The ICR, the Royal Marsden and healthcare company BTG are now looking for partners to fund next-stage clinical trials as soon as possible.
The drug, which will be known as BTG945 going forward, will be highlighted at Asco by the UK’s Department for International Trade, as one of the UK’s best biopharma assets available for licensing or partnering, the ICR said.
Press Association
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks