Exclusive: Three-parent IVF treatment to be legal within weeks
The regulations are expected to be voted on before the general election
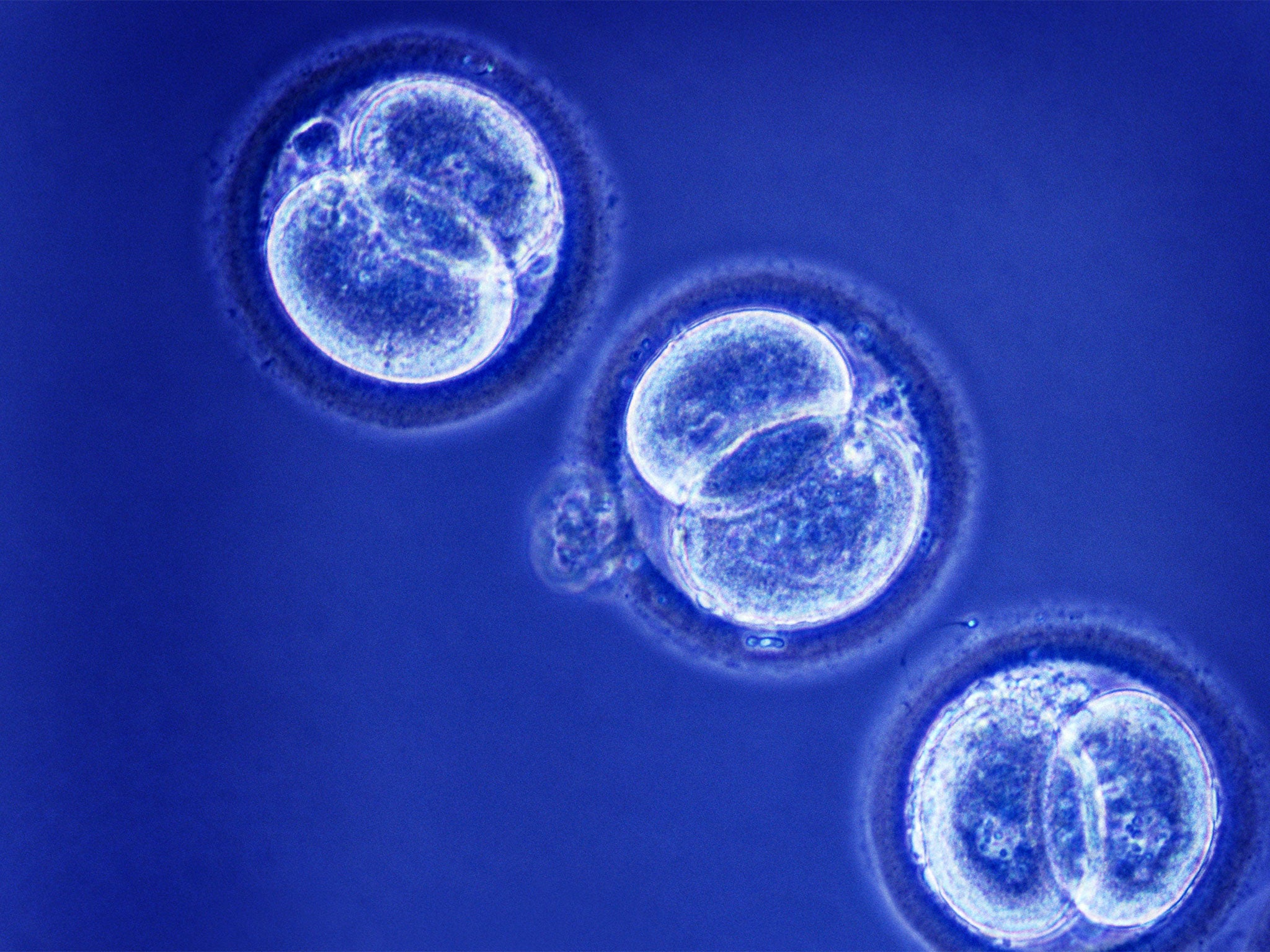
Controversial regulations effectively legalising so-called “three-parent” IVF babies are expected to be debated and voted on by MPs before the general election, and could even be passed within weeks.
Although no timetable for a parliamentary debate on the mitochondrial donation technique has so far been scheduled, the Department of Health confirmed to The Independent it is likely to take place within the next few weeks and almost certainly ahead of the election in May – despite safety concerns.
Mitochondrial donation uses genetic material from the eggs of two women, combined with the sperm of a man, to produce IVF embryos that are free of serious mitochondrial diseases, which currently affect about one in every 6,500 children. Supporters argue it will help women carrying mutations in their mitochondria – the tiny “power packs” of the cells – from passing on the inherited conditions to their children, as the 37 genes of the mitochondria are inherited maternally.
The Department of Health said the technique is a form of germ-line therapy that will affect future generations but has dismissed suggestions it also amounts to genetic modification of embryos with “three biological parents”. Opponents argue the technique is unsafe and could lead to a “slippery slope” of designer children.
The US Food and Drug Administration has said it would take at least another two years to carry out the necessary safety studies before the first American clinical trials on mitochondrial donation, but scientists in Britain have said they could carry out the procedure as early as this year if legislation is passed.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks