Scientists' ability to 'grow' living organs boosts patient transplant hopes
Development that could one day be used to provide replacement organs for people with weakened immune systems
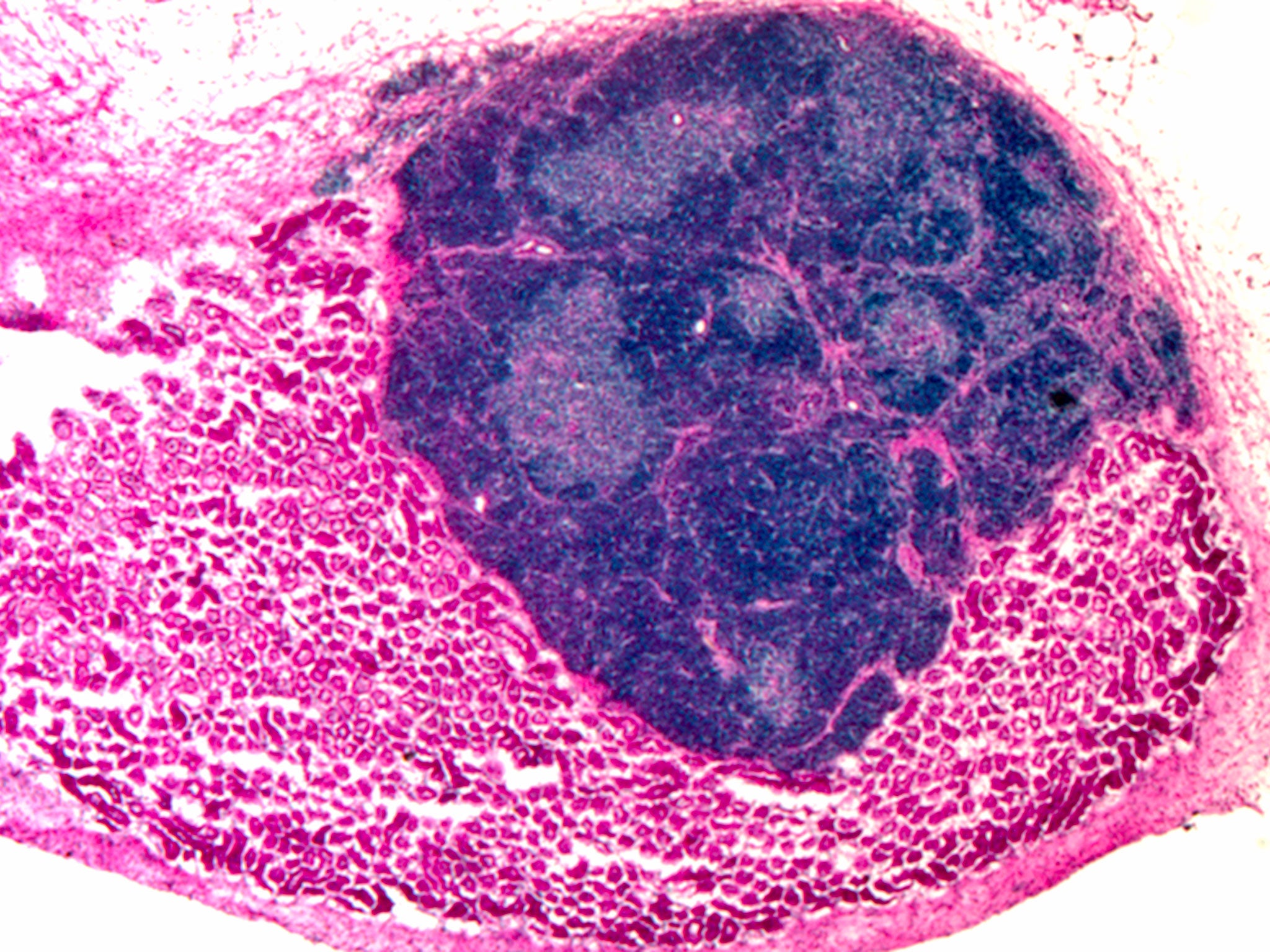
Scientists have created the first functional organ in a living animal from reprogrammed cells in a development that could one day be used to provide replacement organs for people with weakened immune systems.
The thymus organ, a vital immune-system “nerve centre” near the heart, was initially grown in a laboratory from connective-tissue cells. It was then transplanted into laboratory mice, where it continued to grow and develop into a fully functional organ, the researchers said.
It is believed to be the first time that scientists have strung several technologies together to produce a working organ from stem cells which has been transferred into a living animal. It could lead to the transplant of “made-to-order” organs grown from a patient’s own skin cells, though such a breakthrough could take another 10 years and millions of pounds of research.
However, Paolo De Coppi, an expert on regenerative medicine at the Institute of Child Health at Great Ormond Street Hospital in London, suggested an earlier time frame is possible. “Engineering of relatively simple organs has already been adopted for a small number of patients and it is possible that within the next five years, more complex organs will be engineered for patients using specialised cells derived from stem cells in a similar way as outlined in this [study],” he said.
“We’ve managed to produce an artificial cell type which when transplanted can form a fully organised and functional organ. This is an important step towards the goal of generating a clinically useful artificial thymus in the lab,” said Professor Clare Blackburn of the Medical Research Council Centre for Regenerative Medicine at Edinburgh University.
The thymus is a vital part of the immune system because it produces T-cells, which constantly guard the body against invading microbes, viruses and even rogue cancer cells.
People who lack a healthy thymus can be treated with infusions of immune cells or a transplant operation but there is a lack of donors and there are problems with matching tissue types. Being able to create thymus organs from a patient’s own cells would overcome the problem of organ rejection and supply, the researchers said.
“The ability to grow replacement organs from cells in the lab is one of the ‘holy grails’ in regenerative medicine. But the size and complexity of lab-grown organs has so far been limited,” Professor Blackburn added.
Rob Buckle, the head of regenerative medicine at the Medical Research Council, said: “Growing ‘replacement parts’ for damaged tissue could remove the need to transplant whole organs from one person to another, which has many drawbacks, not least a critical lack of donors.”
The thymus organs created by the scientists were able to produce T-cells, a type of white blood cell, in the laboratory and displayed the hallmarks of a fully developed and functional organ.
The scientists started out by taking mouse fibroblasts and inducing them with a “transcription factor” that stimulated their development into thymic epithelial cells. After mixing these cells with other thymus cells in the laboratory, rudimentary thymus organs began to develop. These were then transplanted on to the kidneys of living mice.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies